Long-term potentiation (LTP) and long-term depression (LTD)
Both long-term potentiation (LTP) and long-term depression (LTD) are forms of synaptic plasticity, which refers to the ability of synapses to change their strength over time. These processes are crucial for learning and memory, as they allow the brain to adapt to new information and experiences. Since we are often talking about both processes in the context of computational neuroscience, I thought it would be useful to provide a brief overview of biological mechanisms underlying these processes and their significance in the brain. While the actual mechanisms underlying learning and memory and the role of LTP and LTD are far more complex and still subject of ongoing research, this post aims to provide a general introduction to the current understanding of these processes.
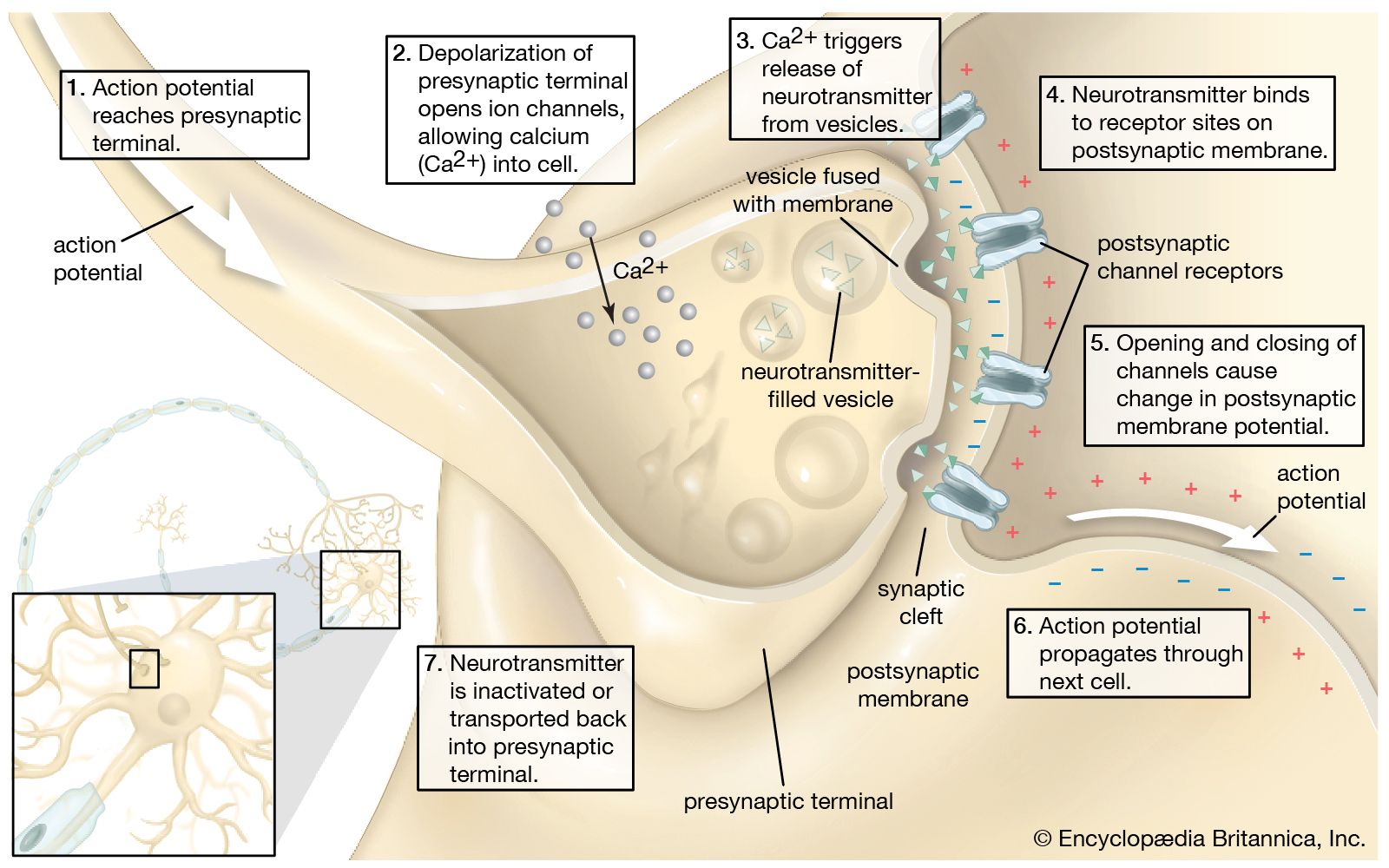 Sketch of the chemical transmission of a nerve impulse at the synapse. The arrival of the nerve impulse at the presynaptic terminal stimulates the release of neurotransmitters into the synaptic gap. The binding of neurotransmitters to receptors on the postsynaptic membrane stimulates the regeneration of the action potential in the postsynaptic neuron. Long-term potentiation (LTP) can enhance the strength of synaptic connections, while long-term depression (LTD) can weaken them. Source: Encyclopædia Britannicaꜛ (license: allowed for display, reproduction, print or download on the services only for personal, non-commercial use)
Sketch of the chemical transmission of a nerve impulse at the synapse. The arrival of the nerve impulse at the presynaptic terminal stimulates the release of neurotransmitters into the synaptic gap. The binding of neurotransmitters to receptors on the postsynaptic membrane stimulates the regeneration of the action potential in the postsynaptic neuron. Long-term potentiation (LTP) can enhance the strength of synaptic connections, while long-term depression (LTD) can weaken them. Source: Encyclopædia Britannicaꜛ (license: allowed for display, reproduction, print or download on the services only for personal, non-commercial use)
Long-term potentiation (LTP)
Long-term potentiation (LTP) is a process by which the strength of synaptic connections between neurons is increased. It is a key mechanism underlying learning and memory in the brain. LTP is typically induced by repeated stimulation of a synapse, leading to long-lasting changes in synaptic strength. The process of LTP involves both presynaptic and postsynaptic changes that enhance the communication between neurons.
The following sketch illustrates the biological process of LTP between two neurons:
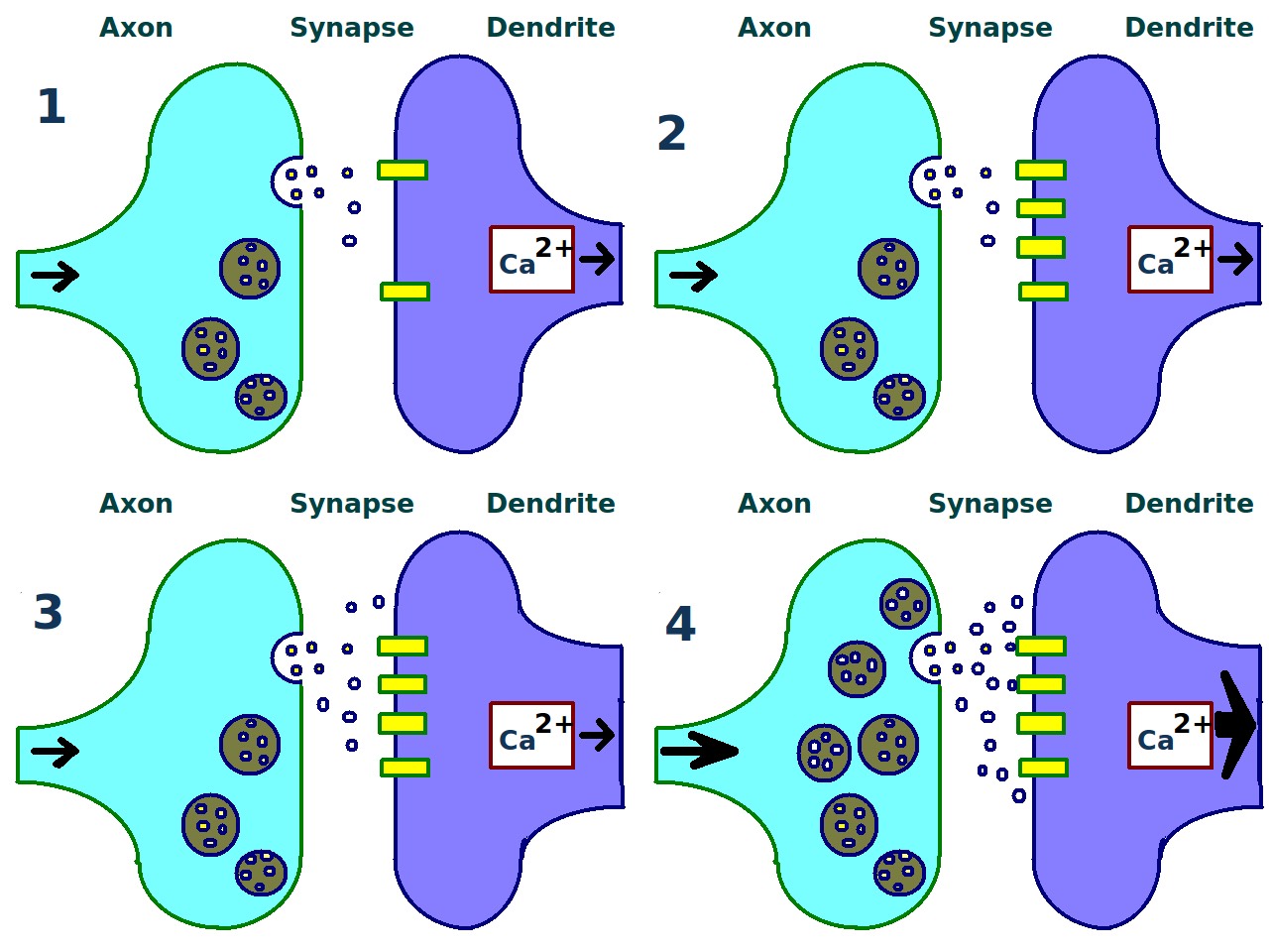 Sketch of a presynaptic axon terminal and a postsynaptic synapse illustrating the biological process of long-term potentiation (LTP) between to neurons. Source: Wikimedia Commonsꜛ (license: Creative Commons CC0 1.0 Universal Public Domain Dedication)
Sketch of a presynaptic axon terminal and a postsynaptic synapse illustrating the biological process of long-term potentiation (LTP) between to neurons. Source: Wikimedia Commonsꜛ (license: Creative Commons CC0 1.0 Universal Public Domain Dedication)
The key steps illustrated include:
1. Repeated Stimulation: A dendritic synapse is repeatedly stimulated (or activated) by neurotransmitters from a presynaptic axon terminal.
2. Postsynaptic Changes: This repeated stimulation causes an increase in the number of postsynaptic receptors (e.g., AMPA receptors) inserted into the postsynaptic membrane, enhancing its sensitivity.
3. Presynaptic Changes: Concurrently, the presynaptic neuron increases the release of neurotransmitters in response to stimuli.
4. Resulting Synaptic Strengthening: The cumulative effect is a stronger synaptic connection between the presynaptic axon terminal and the postsynaptic dendrite, thereby strengthening the communication between the two neurons.
Common neurotransmitters
The communication between neurons is mediated by neurotransmitters, which are chemical messengers that transmit signals across synapses. Several neurotransmitters play a role in modulating synaptic plasticity, including LTP. Here are some of the key neurotransmitters involved in synaptic plasticity:
A common neurotransmitter is glutamate, the main excitatory neurotransmitter in the brain, which binds to postsynaptic receptors such as AMPA and NMDA receptors. Activation of AMPA receptors leads to an influx of sodium ions (Na+), causing depolarization of the postsynaptic membrane. NMDA receptors are typically blocked by magnesium ions (Mg2+) at resting membrane potential. Depolarization (partly through AMPA receptor activation) removes the Mg2+ block, allowing calcium ions (Ca2+) to enter the postsynaptic neuron. This calcium influx is critical for the induction of LTP.
Dopamine can modulate LTP, particularly in brain regions like the hippocampus and striatum. Dopaminergic signaling can enhance or stabilize LTP, making it more likely that synaptic changes will be retained over long periods. A sources for dopamine are dopaminergic neurons, primarily from areas such as the ventral tegmental area (VTA), project to regions like the hippocampus and modulate synaptic plasticity through dopamine release.
Norepinephrine (Noradrenaline) is another neuromodulator that can influence LTP by modulating neuronal excitability and synaptic strength. It often acts through beta-adrenergic receptors to enhance the induction and maintenance of LTP. Released from the locus coeruleus, norepinephrine acts on various brain regions, including the hippocampus.
Serotonin has complex effects on LTP, which can be either facilitative or inhibitory depending on the receptor subtype and brain region involved. It can modulate synaptic plasticity and influence the stability and consolidation of LTP. Serotonergic neurons from the raphe nuclei project broadly throughout the brain, including to the hippocampus.
Acetylcholine is involved in attention and learning processes and can modulate LTP. Cholinergic modulation can enhance synaptic plasticity and the consolidation of LTP. Cholinergic neurons from the basal forebrain project to the hippocampus and cortex, releasing acetylcholine to modulate synaptic function.
Long-term depression (LTD)
Long-term depression (LTD) is a process through which synaptic strength is weakened, contributing to the fine-tuning of neural circuits, learning, and memory.
Key steps include (primarily focusing on the hippocampus):
1. Low-frequency stimulation: LTD is typically induced by low-frequency stimulation (LFS) of presynaptic neurons, often at a frequency of 1 Hz for 10-15 minutes. This type of stimulation is insufficient to remove the magnesium block from NMDA receptors and does not cause significant postsynaptic depolarization.
2. Release of glutamate: During low-frequency stimulation, glutamate is released from the presynaptic terminal and binds to postsynaptic receptors, including NMDA and AMPA receptors.
3. Partial NMDA receptor activation: Unlike LTP, the partial depolarization during LTD induction is not enough to fully unblock NMDA receptors. However, some calcium ions (Ca2+) can still enter the postsynaptic neuron through NMDA receptors, albeit at a lower level than during LTP induction.
4. Calcium signaling: The low levels of Ca2+ influx activate different intracellular signaling pathways compared to those activated during LTP. Specifically, protein phosphatases (eg., PP1, PP2A, and calcineurin) are activated by the modest rise in intracellular calcium. These phosphatases dephosphorylate target proteins, leading to changes in the postsynaptic density.
5. Dephosphorylation of AMPA receptors: Activated phosphatases dephosphorylate AMPA receptors and associated proteins, leading to a reduction in the conductance of these receptors. This results in the internalization of AMPA receptors from the postsynaptic membrane through endocytosis.
6. Reduction in postsynaptic receptors: The removal of AMPA receptors from the postsynaptic membrane decreases the synaptic response to glutamate, effectively weakening the synapse.
7. Synaptic structural changes: Over time, LTD can lead to structural changes in the synapse, such as the reduction of postsynaptic dendritic spine size or even the elimination of synaptic connections.
The key neurotransmitters and molecules, that are involved, are:
- Glutamate: The primary excitatory neurotransmitter involved in both LTP and LTD, binding to NMDA and AMPA receptors.
- NMDA receptors: Partially activated during LTD induction, allowing limited calcium influx.
- Calcium ions (Ca2+): Critical for activating phosphatases that mediate LTD.
- Protein phosphatases: Enzymes such as PP1, PP2A, and calcineurin that dephosphorylate target proteins, leading to AMPA receptor internalization.
- AMPA Receptors: Dephosphorylated and internalized during LTD, reducing synaptic strength.
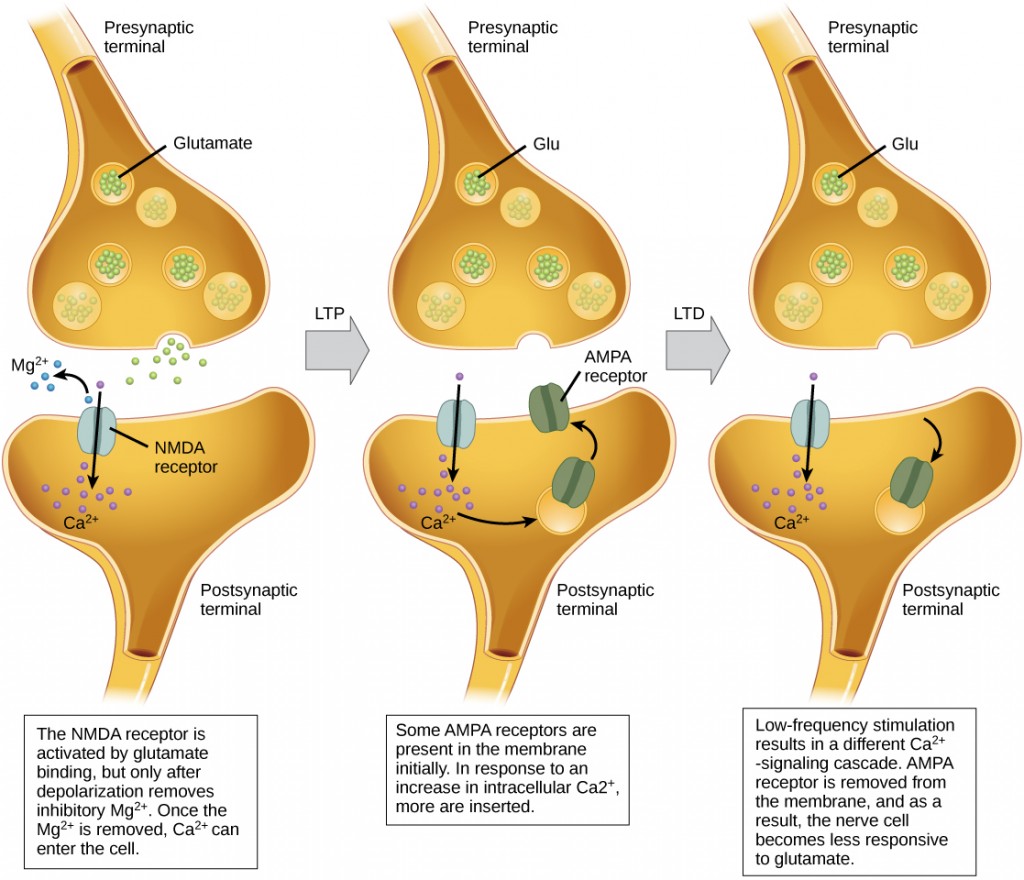 Sketch summarizing the processes of long-term potentiation (LTP) and long-term depression (LTD) at the synapse. Left: During baseline synaptic transmission, glutamate is released from the presynaptic terminal and binds to both AMPA and NMDA receptors on the postsynaptic membrane. At resting membrane potential, NMDA receptors are blocked by Mg2+ ions. Depolarization (often through AMPA receptor activation) removes the Mg2+ block, allowing calcium ions (Ca2+) to enter the postsynaptic cell through the NMDA receptor. This Ca2+ influx is critical for initiating LTP. Middle: In response to this increase in intracellular Ca2+, additional AMPA receptors are inserted into the postsynaptic membrane, increasing the synaptic strength (LTP). Right: Low-frequency stimulation leads to a weaker Ca2+ signal, which triggers the removal of AMPA receptors from the postsynaptic membrane. This results in a reduction of synaptic strength, making the neuron less responsive to glutamate (LTD). Source: E-Campus Ontarioꜛ (license: CC BY-NC-SA 4.0)
Sketch summarizing the processes of long-term potentiation (LTP) and long-term depression (LTD) at the synapse. Left: During baseline synaptic transmission, glutamate is released from the presynaptic terminal and binds to both AMPA and NMDA receptors on the postsynaptic membrane. At resting membrane potential, NMDA receptors are blocked by Mg2+ ions. Depolarization (often through AMPA receptor activation) removes the Mg2+ block, allowing calcium ions (Ca2+) to enter the postsynaptic cell through the NMDA receptor. This Ca2+ influx is critical for initiating LTP. Middle: In response to this increase in intracellular Ca2+, additional AMPA receptors are inserted into the postsynaptic membrane, increasing the synaptic strength (LTP). Right: Low-frequency stimulation leads to a weaker Ca2+ signal, which triggers the removal of AMPA receptors from the postsynaptic membrane. This results in a reduction of synaptic strength, making the neuron less responsive to glutamate (LTD). Source: E-Campus Ontarioꜛ (license: CC BY-NC-SA 4.0)
Cartoon illustrating long-term depression (LTD) (from 2-Minute Neuroscienceꜛ YouTube channel). YouTube video sourceꜛ
Difference between Long-Term and Short-Term Plasticity
In contrast to long-term plasticity, short-term plasticity (STP) refers to transient changes in synaptic strength that occur over a timescale of milliseconds to minutes. These changes in synaptic efficiency are reversible and are generally thought to play a key role in the fine-tuning of neural networks during ongoing neural activity, rather than in long-lasting changes required for memory storage.
STP can manifest in two main forms: short-term potentiation (STP) and short-term depression (STD):
- short-term potentiation (STP) is characterized by a temporary increase in synaptic strength following a brief period of intense presynaptic activity. This increase is often caused by the buildup of presynaptic calcium ions (Ca2+), which enhances neurotransmitter release. Unlike LTP, the synapse’s ability to maintain this enhanced state is short-lived and decays as the calcium concentration returns to baseline.
- short-term depression (STD), on the other hand, is a temporary decrease in synaptic strength, often occurring when neurotransmitter vesicle pools are depleted due to high-frequency stimulation. Synapses need time to recover and replenish their stores of neurotransmitters, and during this recovery period, the synaptic response is weakened.
Thus, key differences between long-term and short-term plasticity are:
- Timescale: The most obvious difference between STP/STD and LTP/LTD lies in the timescale. While LTP and LTD can last from hours to the lifetime of the organism, STP and STD occur over much shorter periods, from a few milliseconds to several minutes, and they dissipate once the activity that triggered them ceases.
- Mechanisms: The molecular mechanisms underlying short-term plasticity are distinct from those of long-term plasticity. STP and STD typically involve presynaptic changes, such as alterations in neurotransmitter release probability, rather than the structural changes to the synapse (e.g., receptor insertion or removal) that are characteristic of LTP and LTD.
- Role in neural circuits: STP and STD are thought to play a critical role in regulating information flow during brief periods of neural activity, allowing synapses to adjust their transmission properties on-the-fly. This rapid form of modulation is particularly important for temporal coding, where the timing of neural signals is crucial for processing information. LTP and LTD, in contrast, are more relevant for the long-term storage of information, such as in learning and memory consolidation.
- Reversibility: Unlike the more permanent changes seen in LTP and LTD, STP and STD are fully reversible, with the synaptic strength returning to baseline after the cessation of the stimulus.
Conclusion
Long-term potentiation (LTP) and long-term depression (LTD) are fundamental processes that underlie synaptic plasticity in the brain. These mechanisms allow the brain to adapt to new information, form memories, and refine neural circuits. The balance between LTP and LTD is crucial for maintaining synaptic homeostasis and proper brain function. Dysregulation of these processes has been implicated in various neurological and psychiatric disorders, highlighting the importance of understanding the molecular and cellular mechanisms underlying synaptic plasticity.
In computational neuroscience, models of LTP and LTD are used to simulate learning and memory processes in artificial neural networks. By incorporating biologically plausible mechanisms of synaptic plasticity, we can develop more realistic models of brain function and behavior.
References
- Robert M. Mulkey, Robert C. Malenka, Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus, 1992, Neuron, Vol. 9, Issue 5, pages 967-975, doi: 10.1016/0896-6273(92)90248-cꜛ
- Serena M. Dudek, Mark F. Bear, Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade., 1992, Proceedings of the National Academy of Sciences, Vol. 89, Issue 10, pages 4363-4367, doi: 10.1073/pnas.89.10.4363ꜛ
- Robert C. Malenka, Mark F. Bear, LTP and LTD, 2004, Neuron, Vol. 44, Issue 1, pages 5-21, doi: 10.1016/j.neuron.2004.09.012ꜛ
- G. L. Collingridge, T. V. P. Bliss, Memories of NMDA receptors and LTP, 1995, Trends in Neurosciences, Vol. 18, Issue 2, pages 54-56, doi: 10.1016/0166-2236(95)80016-Uꜛ
- Adam J. Granger, Roger A. Nicoll, Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on, 2014, Philosophical Transactions of the Royal Society B: Biological Sciences, Vol. 369, Issue 1633, pages 20130136, doi: 10.1098/rstb.2013.0136ꜛ
- Nicoll, A Brief History of Long-Term Potentiation, 2017, Neuron, Vol. 93, Issue 2, pages 281-290, doi: 10.1016/j.neuron.2016.12.015ꜛ
- Zucker, Regehr, Short-Term Synaptic Plasticity, 2002, Annual Review of Physiology, Vol. 64, Issue 1, pages 355-405, doi: 10.1146/annurev.physiol.64.092501.114547ꜛ
- Citri, Malenka, Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms, 2008, Neuropsychopharmacology, Vol. 33, Issue 1, pages 18-41, doi: 10.1038/sj.npp.1301559
- L. F. Abbott, S. B. Nelson, Synaptic plasticity: taming the beast, 2000, Nature neuroscience, doi: 10.1038/81453ꜛ
comments